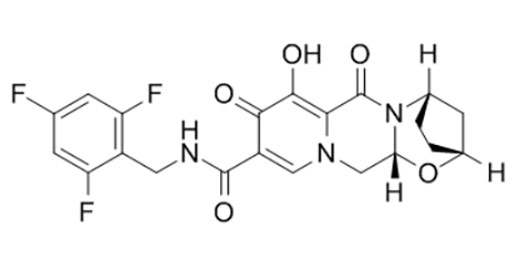
Bictegravir
API’s Name:Bictegravir 1611493-60-7
CAS No.:1611493-60-7
Indication:HIV-1 infection
Innovator:Gilead Sciences
Specification:In-House
Patent Expiry Date(The U.S):Dec. 19, 2033
US DMF:
EU DMF:
CEP:
Only for R&D purpose
Product Detail
Intermediates
Code | CAS | Specification |
BTGM | 1279032-31-3 | In-House |
BTGM2 | 214759-21-4 | In-House |
Description
Bictegravir is a novel, potent inhibitor of HIV-1 integrase with an IC50 of 7.5 nM.
In Vitro
Bictegravir (BIC) inhibits the strand transfer activity with an IC50 of 7.5± 0.3 nM. Relative to its inhibition of strand transfer activity, Bictegravir is a much weaker inhibitor of 3′-processing activity of HIV-1 IN, with an IC50 of 241±51 nM. Bictegravir enhances the accumulation of 2-LTR circles ~5-fold relative to the mock-treated control and reduces the amount of authentic integration products in infected cells by 100-fold. Bictegravir potently inhibits HIV-1 replication in both MT-2 and MT-4 cells with EC50s of 1.5 and 2.4 nM, respectively. Bictegravir exhibits potent antiviral effects in both primary CD4+ T lymphocytes and monocyte-derived macrophages, with EC50s of 1.5±0.3 nM and 6.6±4.1 nM, respectively, which are comparable to values obtained in T-cell lines[1].
MCE has not independently confirmed the accuracy of these methods. They are for reference only.
NCT Number | Sponsor | Condition | Start Date | Phase |
NCT03998176 | University of Nebraska|Gilead Sciences | HIV-1-infection | October 9, 2019 | Phase 4 |
NCT03789968 | Thomas Jefferson University|University of Maryland, College Park|Indiana University Health|The Brooklyn Hospital Center|University of Illinois at Chicago|Nova Southeastern University|University of California, San Francisco | HIV+AIDS | September 1, 2019 | |
NCT04249037 | University of Colorado, Denver|Gilead Sciences | HIV+AIDS | March 1, 2020 | Not Applicable |
NCT04132674 | Vancouver Infectious Diseases Centre | Human Immunodeficiency Virus I Infection|Drug Use | November 26, 2018 | Phase 4 |
NCT04054089 | Cristina Mussini|University of Modena and Reggio Emilia | HIV Infections | September 2019 | Phase 4 |
NCT04155554 | Azienda Ospedaliera Universitaria Senese|Catholic University of the Sacred Heart|Ospedale Policlinico San Martino|Azienda Ospedaliera San Paolo|Ospedale Amedeo di Savoia | HIV-1-infection | January 29, 2020 | Phase 3 |
NCT02275065 | Gilead Sciences | HIV-1 Infection | October 2014 | Phase 1 |
NCT03711253 | University of Southern California | Acute HIV Infection | October 14, 2019 | Phase 4 |
NCT02400307 | Gilead Sciences | HIV | April 17, 2015 | Phase 1 |
NCT03499483 | Fenway Community Health | HIV Prevention | January 24, 2019 | Phase 4 |
NCT03502005 | Midland Research Group, Inc.|Gilead Sciences | Human Immunodeficiency Virus | March 1, 2018 | Phase 4 |
Chemical structure