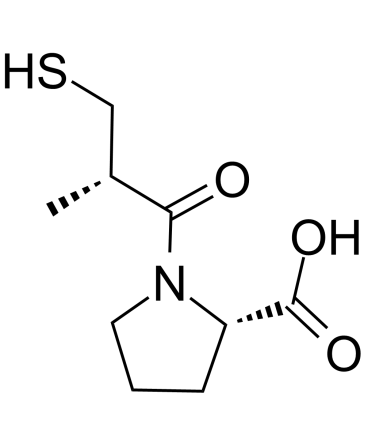
Captopril
API’s Name:Captopril
CAS No.:62571-86-2
Indication:Hypertension
Innovator:
Specification:USP/EP
US DMF:√
EU DMF:√
CEP:
Intermediates
Product Detail
Description
Captopril (SQ-14534) is a potent, competitive inhibitor of angiotensin-converting enzyme (ACE).
In Vitro
Captopril (SQ-14534) has been shown to have similar morbidity and mortality benefits to those of diuretics and beta-blockers in hypertensive patients. Captopril (SQ-14534) has been shown to delay the progression of diabetic nephropathy, and enalapril and lisinopril prevent the development of nephropathy in normoalbuminuric patients with diabetes[1]. An equimolar ratio of the cis and trans states of Captopril (SQ-14534) exists in solution and that the enzyme selects only the trans state of the inhibitor that presents architectural and stereoelectronic complementarity with its substrate binding groove[2].
MCE has not independently confirmed the accuracy of these methods. They are for reference only.
Clinical Trial
NCT Number | Sponsor | Condition | Start Date | Phase |
NCT03179163 | Penn State University|National Heart, Lung, and Blood Institute (NHLBI) | Hypertension,Essential | July 20, 2016 | Phase 1|Phase 2 |
NCT03660293 | Tanta University | Diabetes Mellitus, Type 1 | April 1, 2017 | Not Applicable |
NCT03147092 | Centro Neurológico de Pesquisa e Reabiitação, Brazil | Hypertension|Blood Pressure | February 1, 2018 | Early Phase 1 |
NCT00252317 | Rigshospitalet, Denmark | Aortic Stenosis | November 2005 | Phase 4 |
NCT02217852 | West China Hospital | Hypertension | August 2014 | Phase 4 |
NCT01626469 | Brigham and Women´s Hospital | Type 2 Diabetes Mellitus | May 2012 | Phase 1|Phase 2 |
NCT00391846 | AstraZeneca | Heart Failure|Ventricular Dysfunction, Left | October 2006 | Phase 4 |
NCT00240656 | Hebei Medical University | Hypertension, Pulmonary | October 2005 | Phase 1 |
NCT00086723 | Northwestern University|National Cancer Institute (NCI) | Unspecified Adult Solid Tumor, Protocol Specific | July 2003 | Phase 1|Phase 2 |
NCT00663949 | Shiraz University of Medical Sciences | Diabetic Nephropathy | February 2006 | Phase 2|Phase 3 |
NCT01437371 | University Hospital, Clermont-Ferrand|Servier|LivaNova | Heart Failure | August 2011 | Phase 3 |
NCT04288700 | Ain Shams University | Infantile Hemangioma | October 1, 2019 | Phase 4 |
NCT00223717 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension | January 2001 | Phase 1 |
NCT02770378 | University of Ulm|Reliable Cancer Therapies|Anticancer Fund, Belgium | Glioblastoma | November 2016 | Phase 1|Phase 2 |
NCT01761916 | Instituto Materno Infantil Prof. Fernando Figueira | Preeclampsia | January 2013 | Phase 4 |
NCT01545479 | Instituto de Cardiologia do Rio Grande do Sul | Renal Disease | January 2010 | Phase 4 |
NCT00935805 | Hospital de Clinicas de Porto Alegre|Conselho Nacional de Desenvolvimento Científico e Tecnológico|Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, Brazil | Diabetes Mellitus|Arterial Hypertension | July 2006 | |
NCT00742040 | The Hospital for Sick Children | Heart Disease | August 2008 | Phase 2 |
NCT03613506 | Wuhan University | Radiotherapy Side Effect|Taking Captopril | October 25, 2018 | Phase 2 |
NCT00004230 | Northwestern University|National Cancer Institute (NCI) | Cancer | October 1999 | Phase 3 |
NCT00660309 | Novartis | Type 2 Diabetes Mellitus | April 2008 | Phase 4 |
NCT00292162 | NHS Greater Glasgow and Clyde | Chronic Heart Failure|Atrial Fibrillation | January 2007 | Not Applicable |
NCT01271478 | Coordinación de Investigación en Salud, Mexico | Inflammation|End-stage Renal Disease | August 2009 | Phase 4 |
NCT04193137 | Chongqing Medical University | Primary Aldosteronism | November 30, 2019 | |
NCT00155064 | National Taiwan University Hospital | Hyperaldosteronism | July 2002 | Phase 4 |
NCT01292694 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension|Pure Autonomic Failure|Multiple System Atrophy | March 2011 | Phase 1 |
NCT00917345 | National Taiwan University Hospital|Novartis | Primary Aldosteronism | January 2008 | |
NCT00077064 | Radiation Therapy Oncology Group|National Cancer Institute (NCI)|NRG Oncology | Lung Cancer|Pulmonary Complications|Radiation Fibrosis | June 2003 | Phase 2 |
Storage
Chemicalstructure
Powder | -20°C | 3 years |
4°C | 2 years | |
In solvent | -80°C | 6 months |
-20°C | 1 month |