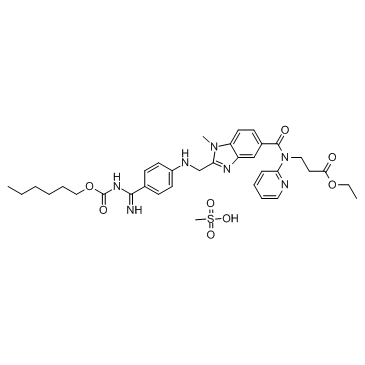
Dabigatran Etexilate Mesylate
API’s Name:Dabigatran Etexilate Mesylate
CAS No.:872728-81-9
Indication:Anticoagulant
Innovator:
Specification:In-House
US DMF:
EU DMF:
CEP:
Only for R&D purpose
Product Detail
Intermediates
Code | CAS | Specification |
DB | 872728-81-9 | In-House |
DB-A | 211915-84-3 | In-House |
DB-SM2 | 212322-56-0 | In-House |
DB-SM1 | 42288-26-6 | In-House |
Description
Dabigatran etexilate mesylate (BIBR 1048MS) is an orally active prodrug of Dabigatran. Dabigatran etexilate mesylate has anticoagulant effects and is used for the prophylaxis of venousthromboembolism and stroke due to atrial fibrillation.
Background
Description: IC50 Value: 4.5nM (Ki); 10nM(Thrombin-induced platelet aggregation) [1] Dabigatran is a reversible and selective, direct thrombin inhibitor (DTI) undergoing advanced clinical development as its orally active prodrug,dabigatran etexilate. in vitro: Dabigatran selectively and reversibly inhibited human thrombin(Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC(50): 10 nM), while showing no inhibitory effect on other platelet-stimulating agents.Thrombin generation in platelet-poor plasma (PPP), measured as the endogenous thrombin potential (ETP) was inhibited concentration-dependently (IC(50): 0.56 microM). Dabigatran demonstrated concentration-dependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 microM, respectively [1]. in vivo: Dabigatran prolonged the aPTT dose-dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dose- and time-dependent anticoagulant effects were observed with dabigatran etexilate administered orally to conscious rats (10, 20 and 50 mg/kg) or rhesus monkeys (1, 2.5 or 5 mg/kg), with maximum effects observed between 30 and 120 min after administration, respectively [1]. Patients treated with dabigatran etexilate experienced fewer ischaemic strokes (3.74 dabigatran etexilate vs 3.97 warfarin) and fewer combined intracranial haemorrhages and haemorrhagic strokes (0.43 dabigatran etexilate vs 0.99 warfarin) per 100 patient-years [2]. Clinical trial: An Evaluation of the Pharmacokinetics and Pharmacodynamics of Oral Dabigatran Etexilate in Hemodialysis Patients . Phase1
Storage
| Powder | -20°C | 3 years |
4°C | 2 years | |
| In solvent | -80°C | 6 months |
-20°C | 1 month |
Clinical Trial
NCT Number | Sponsor | Condition | Start Date | Phase |
NCT02170792 | Boehringer Ingelheim | Healthy | February 2001 | Phase 1 |
NCT02170974 | Boehringer Ingelheim | Healthy | July 2004 | Phase 1 |
NCT02170831 | Boehringer Ingelheim | Healthy | May 1999 | Phase 1 |
NCT02170805 | Boehringer Ingelheim | Healthy | April 2001 | Phase 1 |
NCT02170610 | Boehringer Ingelheim | Healthy | March 2002 | Phase 1 |
NCT02170909 | Boehringer Ingelheim | Healthy | December 2004 | Phase 1 |
NCT02171000 | Boehringer Ingelheim | Healthy | April 2005 | Phase 1 |
NCT02170844 | Boehringer Ingelheim | Healthy | June 2004 | Phase 1 |
NCT02170584 | Boehringer Ingelheim | Healthy | January 2001 | Phase 1 |
NCT02170935 | Boehringer Ingelheim | Venous Thromboembolism | April 2002 | Phase 2 |
NCT02170636 | Boehringer Ingelheim | Healthy | January 2002 | Phase 1 |
NCT02170766 | Boehringer Ingelheim | Healthy | October 2000 | Phase 1 |
NCT02171442 | Boehringer Ingelheim | Healthy | April 2002 | Phase 1 |
NCT02170896 | Boehringer Ingelheim | Healthy | October 2001 | Phase 1 |
NCT02173730 | Boehringer Ingelheim | Healthy | November 2002 | Phase 1 |
NCT02170623 | Boehringer Ingelheim | Healthy | February 2002 | Phase 1 |
NCT02170116 | Boehringer Ingelheim | Healthy | November 1998 | Phase 1 |
NCT02170597 | Boehringer Ingelheim | Healthy | August 2003 | Phase 1 |
NCT01225822 | Boehringer Ingelheim | Venous Thromboembolism | November 2002 | Phase 2 |
NCT02170701 | Boehringer Ingelheim | Venous Thromboembolism | October 2000 | Phase 2 |
NCT02170740 | Boehringer Ingelheim | Healthy | November 1999 | Phase 1 |
NCT02170922 | Boehringer Ingelheim | Healthy | July 1999 | Phase 1 |
Chemical structure