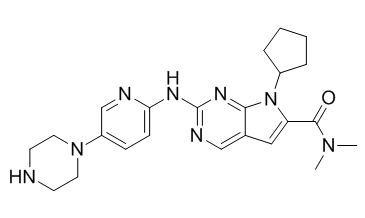
Ribociclib
API’s Name:Ribociclib
CAS No.:1374639-75-4
Indication:HR-positive, HER2-negative Andvance ormetastaticbreast Cancers
Innovator:Novartis Pharmaceuticals
Specification:In-House
US DMF:
EU DMF:
CEP:
Patent Expiry Date(The U.S):Jun.27, 2028
Only for R&D purpose
Product Detail
Intermediates
Code | CAS | Specification |
Ribociclib | 1374639-75-4 | In-House |
Description
Ribociclib (LEE01) is a highly specific CDK4/6 inhibitor with IC50 values of 10 nM and 39 nM, respectively, and is over 1,000-fold less potent against the cyclin B/CDK1 complex.
In Vitro
Treating a panel of 17 neuroblastoma cell lines with Ribociclib (LEE011) across a four-log dose range (10 to 10,000 nM). Treatment with Ribociclib significantly inhibits substrate adherent growth relative to the control in 12 of the 17 neuroblastoma cell lines examined (mean IC50=306±68 nM, considering sensitive lines only, where sensitivity is defined as an IC50 of less than 1 μM. Ribociclib treatment of two neuroblastoma cell lines (BE2C and IMR5) with demonstrated sensitivity to CDK4/6 inhibition results in a dose-dependent accumulation of cells in the G0/G1 phase of the cell cycle. This G0/G1 arrest becomes significant at Ribociclib concentrations of 100 nM (p=0.007) and 250 nM (p=0.01), respectively.
CB17 immunodeficient mice bearing BE2C, NB-1643 (MYCN amplified, sensitive in vitro), or EBC1 (non-amplified, resistant in vitro) xenografts are treated once daily for 21 days with Ribociclib (LEE011; 200 mg/kg) or with a vehicle control. This dosing strategy is well tolerated, as no weight loss or other signs of toxicity are observed in any of the xenograft models. Tumor growth is significantly delayed throughout the 21 days of treatment in mice harboring the BE2C or 1643 xenografts (both, p<0.0001), although growth resumed post-treatment.
Storage
| Powder | -20°C | 3 years |
4°C | 2 years | |
| In solvent | -80°C | 6 months |
-20°C | 1 month |
Chemical structure