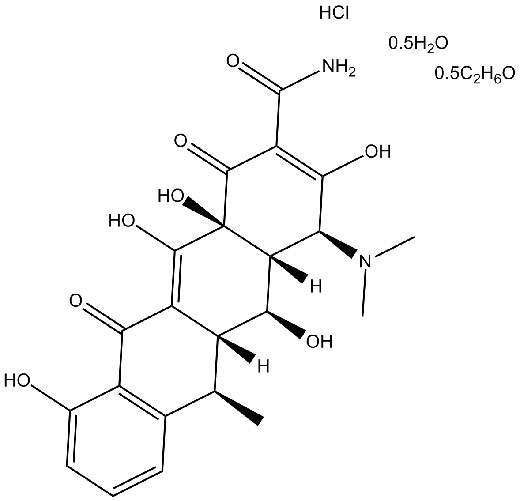
Doxycycline Hyclate
API’s Name:Doxycycline Hyclate
CAS No.:24390-14-5
Indication:Antibiotics
Innovator:
Specification:USP/EP
US DMF:13771
EU DMF:√
CEP:
Only for R&D purpose
Intermediates
Product Detail
Background
Doxycycline hyclate is an antibiotic [1].
Doxycycline hyclate is a derivative of tetracycline and possesses the activities of anti-inflammatory and antimicrobial. Doxycycline inhibits dengue virus replication in vitro with a temperature-dependent manner. The IC50 value is 52.3μM at 37°C and 26.7μM at 40°C. It inhibits the dengue virus via inhibiting the NS2B-NS3 serine protease of the virus. 60μM doxycycline reduces the CPE of the DNEV2-infected cells [1].
Doxycycline is found to be an inhibitor of MMP. Doxycycline treatment reduces MMP-8 and -9 levels and inhibits expression of tissue MMP-2 and MMP-9. Moreover, treatment with doxycycline significantly reduces the incidence of intracranial aneurysms. Doxycycline has also been reported to be an anti-inflammatory agent based on its inhibition of matrix metalloproteinases. In addition, doxycycline has potent antimalarial activity with IC50 value of 320nM at 96h in vitro [2, 3].
References:
[1] Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014 Apr;159(4):711-8.
[2] Maradni A, Khoshnevisan A, Mousavi SH, Emamirazavi SH, Noruzijavidan A. Role of matrix metalloproteinases (MMPs) and MMP inhibitors on intracranial aneurysms: a review article. Med J Islam Repub Iran. 2013 Nov;27(4):249-254.
[3] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nelson ML. In vitro and in vivo antimalarial efficacies of optimized tetracyclines. Antimicrob Agents Chemother. 2013 Jul;57(7):3131-6.
Description
Doxycycline (hyclate) (Doxycycline hydrochloride hemiethanolate hemihydrate), an antibiotic, is an orally active and broad-spectrum metalloproteinase (MMP) inhibitor[1].
Clinical Trial
NCT Number | Sponsor | Condition | Start Date | Phase |
NCT00246324 | Louisiana State University Health Sciences Center Shreveport|Biogen | Multiple Sclerosis | December 2003 | Phase 4 |
NCT00910715 | University Medical Centre Ljubljana | Erythema Chronicum Migrans | June 2009 | Not Applicable |
NCT00243893 | University of California, San Francisco|National Institute of Neurological Disorders and Stroke (NINDS) | Aneurysms|Arteriovenous Malformations | July 2004 | Phase 1 |
NCT00126399 | CollaGenex Pharmaceuticals | Rosacea | June 2004 | Phase 3 |
NCT01318356 | Radboud University|ZonMw: The Netherlands Organisation for Health Research and Development | Q Fever|Fatigue Syndrome, Chronic|Coxiella Infection | April 2011 | Phase 4 |
NCT00177333 | University of Pittsburgh | Abortion, Induced|Vomiting | September 2005 | Phase 4 |
NCT00007735 | US Department of Veterans Affairs|Pfizer|United States Department of Defense|VA Office of Research and Development | Persian Gulf Syndrome|Mycoplasma Infections | January 1999 | Phase 3 |
NCT00351273 | University of South Florida|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) | Arthritis, Reactive|Reiter Disease | May 2006 | Phase 3 |
NCT00469261 | Careggi Hospital | Myocardial Infarction|Left Ventricular Remodeling | May 2007 | Phase 2 |
NCT00547170 | University of Pittsburgh|Tu Du Hospital | Endometritis | January 2007 | Phase 4 |
NCT01475708 | University Medical Centre Ljubljana | Lyme Borreliosis | May 2011 | |
NCT01368341 | Morten Lindbaek|Norwegian Institute of Public Health|Sorlandet Hospital HF|Norwegian University of Life Sciences|University of Oslo | Erythema Migrans|Erythema Chronicum Migrans|Borreliosis|Lyme Disease|Early Lyme Disease | June 2011 | Phase 4 |
NCT02538224 | Islamic Azad University, Tehran | Chronic Periodontitis | July 2013 | Phase 2|Phase 3 |
NCT00066027 | University of Nebraska|National Institute of Dental and Craniofacial Research (NIDCR) | Periodontitis | June 2002 | Phase 3 |
NCT00376493 | Hospital de Clinicas de Porto Alegre | Septic Abortion | May 2006 | Phase 4 |
NCT03448731 | Fundacion CRIS de Investigación para Vencer el Cáncer|Amgen|Apices Soluciones S.L. | Skin Toxicity | May 10, 2018 | Phase 2 |
NCT00989742 | University of Nottingham | Lymphangioleiomyomatosis|Tuberous Sclerosis | July 2009 | Phase 4 |
NCT01438515 | Horizon Health Network | Methicillin-resistant Staphylococcus Aureus | August 2008 | Not Applicable |
NCT02929121 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema|Lymphatic Filariasis|Filariasis | January 15, 2019 | Phase 3 |
NCT00952861 | Odense University Hospital|Kolding Sygehus|Svendborg Hospital|Fredericia Hospital|Naestved Hospital|Hillerod Hospital, Denmark|Region Syddanmark|Danmarks Lungeforening|Danish National Research Foundation | Pulmonary Disease, Chronic Obstructive | October 2009 | Phase 4 |
NCT00138801 | Sorlandet Hospital HF | Lyme Neuroborreliosis | March 2004 | Phase 3 |
NCT00942006 | University Medical Centre Ljubljana | Suspected Early Lyme Neuroborreliosis | July 2009 | Not Applicable |
NCT02713607 | University of California, Davis | Acne Vulgaris | March 2016 | Phase 1|Phase 2 |
NCT00560703 | Galderma | Blepharitis|Meibomianitis|Dry Eye | November 2007 | Phase 2 |
NCT01014260 | Johns Hopkins University | Cardiovascular Disease | September 2010 | Phase 4 |
NCT00000938 | National Institute of Allergy and Infectious Diseases (NIAID) | Lyme Disease | Phase 3 | |
NCT01398072 | University College, London|Royal Free Hampstead NHS Trust|University of Cambridge|National Institute for Health Research, United Kingdom | Chronic Obstructive Pulmonary Disease (COPD). | December 2011 | Phase 3 |
NCT03479502 | Vanderbilt University Medical Center|Orthopedic Research and Education Foundation | Adhesive Capsulitis|Adhesive Capsulitis of Unspecified Shoulder|Frozen Shoulder | January 5, 2018 | Phase 4 |
NCT02929134 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema|Lymphatic Filariasis|Filariasis | February 16, 2018 | Phase 3 |
NCT00480532 | Oregon Health and Science University | Contraceptives, Oral | May 2007 | Not Applicable |
NCT01594827 | Johns Hopkins University|Case Western Reserve University|Cystic Fibrosis Foundation | Cystic Fibrosis | October 2012 | Phase 2 |
NCT01744093 | Weill Medical College of Cornell University|National Heart, Lung, and Blood Institute (NHLBI) | HIV|Chronic Obstructive Pulmonary Disease (COPD)|Emphysema | July 17, 2014 | Not Applicable |
NCT03530319 | National Taiwan University Hospital | Pneumonia, Mycoplasma | November 10, 2018 | Not Applicable |
NCT04167085 | University of California, Los Angeles | Epistaxis | December 18, 2017 | Phase 4 |
NCT01411202 | Ottawa Hospital Research Institute | Malignant Pleural Effusion | June 2011 | Phase 2 |
NCT01474590 | Galderma | Acne | November 2011 | Phase 3 |
NCT00649571 | Mylan Pharmaceuticals | Healthy | July 2005 | Phase 1 |
NCT02899000 | Galderma Laboratories, L.P. | Acne Vulgaris | July 29, 2016 | Phase 4 |
NCT00538967 | Leiden University Medical Center | Aortic Aneurysm, Abdominal | May 2002 | Phase 2 |
NCT00439400 | Alacrity Biosciences, Inc. | Dry Eye | February 2007 | Phase 2 |
NCT00917553 | Thomas Gardner|Penn State University|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetic Retinopathy | July 2009 | Phase 2 |
NCT00495313 | CollaGenex Pharmaceuticals | Rosacea | March 2007 | Phase 4 |
NCT01855360 | Brigham and Women´s Hospital | Amyloidosis; Heart (Manifestation)|Senile Cardiac Amyloidosis | June 2013 | Phase 1|Phase 2 |
NCT00419848 | Shahid Beheshti University of Medical Sciences | Acne | August 2006 | Phase 2 |
NCT03532464 | University Hospital, Bordeaux|USC EA 3671 Infections humaines à mycoplasmes et à chlamydiae | Chlamydia Trachomatis Infection|Vaginal Infection|Anal Infection | July 1, 2018 | Phase 4 |
NCT02756403 | Medstar Health Research Institute|Society of Family Planning | First Trimester Abortion | March 2016 | Not Applicable |
NCT00353158 | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|National Institutes of Health Clinical Center (CC) | Healthy Volunteers|Fungal Infections|Bacterial Infections | July 11, 2006 | Phase 1 |
NCT01317433 | Institut Cancerologie de l´Ouest | Colorectal Cancer Metastatic|Skin Toxicity | December 2010 | Phase 3 |
NCT01658995 | Petra M. Casey|Mayo Clinic | ESI-related Bleeding | September 13, 2012 | Phase 3 |
NCT03968562 | State University of New York - Downstate Medical Center | Hives | May 15, 2019 | Phase 2 |
NCT02569437 | Icahn School of Medicine at Mount Sinai | Polyp of Nasal Sinus | September 2014 | Phase 2 |
NCT01198509 | NYU Langone Health|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|Memorial Sloan Kettering Cancer Center | Rheumatoid Arthritis|Psoriatic Arthritis|Periodontal Disease | January 2010 | Not Applicable |
NCT01163994 | University Medical Centre Ljubljana | Multiple Erythema Migrans | June 2010 | Not Applicable |
NCT02388477 | Milton S. Hershey Medical Center | Rotator Cuff Injury | Not Applicable | |
NCT01010295 | International Extranodal Lymphoma Study Group (IELSG) | Non-Hodgkin Lymphoma | September 2006 | Phase 2 |
NCT00775918 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Healthy | June 2005 | Not Applicable |
NCT04050540 | University of Washington|Kenya Medical Research Institute|Kenya National AIDS & STI Control Programme|University of California, San Francisco|National Institute of Allergy and Infectious Diseases (NIAID) | HIV Infections|HIV+AIDS|Neisseria Gonorrheae Infection|Chlamydia Trachomatis Infection|Syphilis Infection | February 5, 2020 | Phase 4 |
NCT02562651 | Russian Academy of Medical Sciences | Vascular Diseases|Cardiovascular Diseases|Acute Myocardial Infarction | February 2014 | Phase 2|Phase 3 |
NCT00001101 | National Institute of Allergy and Infectious Diseases (NIAID) | Lyme Disease | Phase 3 | |
NCT00340691 | National Institute of Allergy and Infectious Diseases (NIAID)|National Institutes of Health Clinical Center (CC) | Mansonella Perstans Infection|Mp Microfilaremia | December 6, 2004 | Phase 2 |
NCT01112059 | University of Alabama at Birmingham|Cystic Fibrosis Foundation | Cystic Fibrosis | November 2008 | Not Applicable |
NCT00652704 | Par Pharmaceutical, Inc.|Anapharm | To Determine Bioequivalence Under Fed Conditions | July 1999 | Phase 1 |
NCT01783860 | Tehran University of Medical Sciences | Posterior Blepharitis | January 2013 | Phase 2 |
NCT02564471 | State University of New York - Upstate Medical University|Walter Reed Army Institute of Research (WRAIR)|Kansas State University | Rabies | April 2016 | Phase 4 |
NCT04206631 | Indonesia University | Acne Vulgaris | April 1, 2015 | Phase 1 |
NCT03956446 | University Medical Centre Ljubljana|University of Ljubljana School of Medicine, Slovenia | Tick Borne Encephalitis | September 1, 2014 | Not Applicable |
NCT03960411 | Felix Chikita Fredy, MD|National Cardiovascular Center Harapan Kita Hospital Indonesia|Indonesia University | ST Elevation Myocardial Infarction|Anterior Wall Myocardial Infarction|Heart Failure|Remodeling, Ventricular | May 25, 2019 | Phase 3 |
NCT00322465 | National Institute of Allergy and Infectious Diseases (NIAID) | Urethritis | November 2006 | Phase 2 |
NCT01375491 | University of California, San Diego|Ruth L. Kirschstein National Research Service Award|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)|National Center for Research Resources (NCRR) | Type 2 Diabetes|Obesity | October 2009 | Phase 4 |
NCT03478436 | Medical University of Vienna|Dr. Reddy´s Laboratories Limited | Rosacea | July 2016 | Phase 1 |
NCT01207739 | Radboud University|Sint Maartenskliniek|ZonMw: The Netherlands Organisation for Health Research and Development | Lyme Disease|Borrelia Infection | September 2010 | Phase 4 |
NCT00939562 | Pfizer | Bacterial Infection | November 2008 | Phase 4 |
NCT03608774 | National Institute of Allergy and Infectious Diseases (NIAID) | Anal Chlamydia Infection | June 26, 2018 | Phase 4 |
NCT02281643 | Kwame Nkrumah University of Science and Technology|University of Bonn|Heinrich-Heine University, Duesseldorf | Mansonella Perstans Infection|Buruli Ulcer|Tuberculosis|Co-infection | October 2014 | Phase 2 |
NCT00066066 | The Forsyth Institute|National Institute of Dental and Craniofacial Research (NIDCR) | Periodontitis|Periodontal Diseases | July 2003 | Phase 2 |
NCT01798225 | Medical University of South Carolina|National Center for Research Resources (NCRR) | Periodontal Disease|Type 2 Diabetes Mellitus | December 2007 | Phase 4 |
NCT00612573 | Warner Chilcott | Acne Vulgaris | February 2008 | Phase 2 |
NCT01631617 | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|National Institutes of Health Clinical Center (CC) | Eczema|Dermatitis|Skin Diseases, Genetic|Dermatitis, Atopic|Skin Diseases | September 18, 2012 | Phase 2 |
NCT03173053 | Radboud University|ZonMw: The Netherlands Organisation for Health Research and Development|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Aalborg University Hospital|Rigshospitalet, Denmark | Staphylococcus Aureus|Motility Disorder | February 8, 2018 | Not Applicable |
NCT00715858 | McMaster University|The Physicians´ Services Incorporated Foundation | Alzheimer´s Disease | May 2008 | Phase 3 |
NCT03584919 | University Medical Centre Ljubljana | Erythema Chronicum Migrans | June 1, 2006 | Not Applicable |
NCT01469585 | University of Hawaii|Charles Drew University of Medicine and Science|Meharry Medical College | Breakthrough Bleeding | November 2011 | Not Applicable |
NCT02759120 | Weill Medical College of Cornell University|Duke Clinical Research Institute|University of Chicago|University of Washington|University of Pittsburgh|National Heart, Lung, and Blood Institute (NHLBI) | Idiopathic Pulmonary Fibrosis | March 22, 2017 | Phase 3 |
NCT02735837 | Amirhossein Farahmand|Islamic Azad University, Tehran | Diabetes Mellitus With Periodontal Disease | January 2015 | Phase 2|Phase 3 |
NCT03655197 | University of California, Davis | Rosacea|Ocular Rosacea|Cutaneous Rosacea | November 2, 2017 | Early Phase 1 |
NCT01188954 | Northwell Health | Seroma | January 2010 | Not Applicable |
NCT00388778 | Shahid Beheshti University of Medical Sciences | Acne|Inflammation | October 2005 | Phase 2|Phase 3 |
NCT01087476 | Metropolitan Autonomous University|Instituto Nacional de Cancerologia de Mexico | Mucositis | May 2010 | Phase 2 |
NCT02174757 | CD Pharma India Pvt. Ltd.|Sree Mookambika Institute of Dental Sciences | Chronic Periodontitis | August 2014 | Phase 3 |
NCT03911440 | National Taiwan University Hospital | Atypical Pneumonia | November 10, 2018 | Not Applicable |
NCT02553083 | Rabin Medical Center | Bacterial Infection Due to Helicobacter Pylori (H. Pylori) | October 22, 2015 | Phase 4 |
NCT04234945 | Ahmadu Bello University Teaching Hospital | Infertility, Female|Pelvic Inflammatory Disease | January 13, 2020 | Not Applicable |
NCT00892281 | Galderma Laboratories, L.P. | Rosacea | April 2009 | Phase 4 |
NCT02913118 | Qingfeng Pharmaceutical Group | Community Acquired Pneumonia | July 2016 | Phase 4 |
NCT04153604 | Methodist Health System | Cirrhosis|Spontaneous Bacterial Peritonitis | November 4, 2019 | |
NCT03153267 | University Medical Centre Ljubljana|University of Ljubljana School of Medicine, Slovenia | Erythema Chronicum Migrans | June 1, 2017 | Not Applicable |
NCT03116659 | James J. Peters Veterans Affairs Medical Center | Lymphoma, T-Cell, Cutaneous | February 1, 2018 | Early Phase 1 |
NCT03401372 | Jian Li|Peking University First Hospital|Chinese PLA General Hospital|Beijing Chao Yang Hospital|West China Hospital Affiliated with Sichuan University|Tongji Hospital Affiliated with Tongji Medical College of HUST|Union Hospital Affiliated with Tongji Medical College of HUST|Shanghai Changzheng Hospital|Nanfang Hospital of Southern Medical University|Peking Union Medical College Hospital | Amyloidosis; Systemic | April 21, 2018 | Not Applicable |
NCT01380496 | Par Pharmaceutical, Inc.|Anapharm | To Determine Bioequivalence Under Fed Conditions | November 1999 | Phase 1 |
NCT03083197 | University of Oxford|Shoklo Malaria Research Unit|Chiangrai Prachanukroh Hospital | Scrub Typhus | October 15, 2017 | Phase 4 |
NCT00237016 | Medical Corps, Israel Defense Force | Relapsing Fever, Tick-Borne|Jarisch Herxheimer Reaction | April 2002 | Phase 2|Phase 3 |
NCT01308619 | Galderma Laboratories, L.P. | Rosacea | April 2011 | Phase 4 |
NCT01198912 | University Hospital, Ghent | Chronic Rhinosinusitis|Nasal Polyps | November 22, 2011 | Phase 2 |
NCT02016365 | Umeå University | Transthyretin Amyloidosis|Cardiomyopathy | February 2012 | Phase 2 |
NCT00783523 | University of California, San Francisco | Arteriovenous Malformations|Cavernous Angiomas|Brain Aneurysms | March 2008 | Phase 1 |
NCT03337932 | University Medical Centre Ljubljana | Erythema Chronicum Migrans | January 1, 2018 | Not Applicable |
NCT00568711 | Dong-Min Kim|Chosun University Hospital | Scrub Typhus | September 2006 | Not Applicable |
NCT01874860 | University of Louisville|James Graham Brown Cancer Center | Colorectal Cancer|Head and Neck Cancer | August 2013 | Phase 2 |
NCT01171859 | IRCCS Policlinico S. Matteo | Transthyretin Amyloidosis | July 2010 | Phase 2 |
NCT01653522 | The Cleveland Clinic | Migraine Disorders|Headache, Migraine|Migraine|Migraine Headache|Migraine With Aura|Migraine Without Aura|Headache Disorders, Primary | July 2012 | Not Applicable |
NCT01820910 | International Extranodal Lymphoma Study Group (IELSG) | Marginal Zone Lymphoma of Ocular Adnexal | March 2013 | Phase 2 |
NCT01323101 | University of Southern California | Cystic Fibrosis | April 2008 | Phase 4 |
NCT00829764 | Teva Pharmaceuticals USA | Healthy | October 2006 | Phase 1 |
NCT01668498 | AIO-Studien-gGmbH | Ras-wildtype Colorectal Cancer | May 2011 | Phase 2 |
NCT01030666 | Peter Eickholz|Heidelberg University|Dr. August Wolff GmbH & Co. KG Arzneimittel|Gaba International AG|Goethe University | Periodontitis | April 2007 | Phase 4 |
NCT00012688 | US Department of Veterans Affairs|Colgate-Periogard-Dentsply|VA Office of Research and Development | Diabetes Mellitus|Poor Glycemic Control|Peridontal Disease | Not Applicable | |
NCT01885910 | Derm Research, PLLC|WFH MEDICAL, LLC | Acne Vulgaris | July 2013 | Phase 4 |
NCT02328469 | University Medical Centre Ljubljana|Slovenian Research Agency|University of Ljubljana School of Medicine, Slovenia|Harvard University | Aseptic Meningitis | June 2014 | |
NCT00355602 | University of Dundee|Tenovus Scotland | Colitis, Ulcerative | July 2006 | Not Applicable |
NCT02606032 | Hamilton Health Sciences Corporation|Hamilton Academic Health Sciences Organization | Ulcerative Colitis | May 2016 | Phase 2 |
NCT01465802 | Pfizer | Non Small Cell Lung Cancer (NSCLC) | December 26, 2011 | Phase 2 |
NCT02623959 | M.D. Anderson Cancer Center | Advanced Cancers|Malignant Pleural Effusions | April 27, 2016 | Phase 4 |
NCT03481972 | IRCCS Policlinico S. Matteo | TTR Cardiac Amyloidosis | April 11, 2018 | Phase 3 |
NCT00428818 | University of Texas Southwestern Medical Center | Infection | August 2005 | Not Applicable |
NCT01935622 | Virginia Commonwealth University | Non-ischemic Cardiomyopathy|Systolic Heart Failure (NYHA II-III) | July 2012 | Phase 2 |
NCT01886560 | Sun Yat-sen University | Eye Burns | September 2013 | Phase 2|Phase 3 |
NCT04239755 | Damanhour University|Tanta University | Traumatic Brain Injury | December 15, 2019 | Phase 4 |
NCT02204254 | Centre Hospitalier Universitaire de Nice | Rosacea | March 2014 | Not Applicable |
NCT00837213 | Stiefel, a GSK Company|GlaxoSmithKline | Acne | August 2007 | Phase 4 |
NCT03115177 | Rush University Medical Center | Osteoarthritis | November 2015 | Not Applicable |
NCT03618108 | Cadrock Pty. Ltd.|Centre for Digestive Diseases, Australia | Coronary Heart Disease|Chlamydophila Pneumoniae Infections | April 4, 2018 | Phase 2 |
NCT03435952 | M.D. Anderson Cancer Center|BioMed Valley Discoveries, Inc|Merck Sharp & Dohme Corp. | Malignant Neoplasm of Breast|Malignant Neoplasms of Digestive Organs|Malignant Neoplasms of Eye Brain and Other Parts of Central Nervous System|Malignant Neoplasms of Female Genital Organs|Malignant Neoplasms of Ill-defined Secondary and Unspecified Sites|Malignant Neoplasms of Independent (Primary) Multiple Sites|Malignant Neoplasms of Lip Oral Cavity and Pharynx|Malignant Neoplasms of Male Genital Organs|Malignant Neoplasms of Mesothelial and Soft Tissue|Malignant Neoplasms of Respiratory and Intrathoracic Organs|Malignant Neoplasms of Thyroid and Other Endocrine Glands|Malignant Neoplasms of Urinary Tract | July 10, 2018 | Phase 1 |
NCT01867294 | Academic and Community Cancer Research United|National Cancer Institute (NCI) | Advanced Malignant Neoplasm|Dermatologic Complication | August 31, 2012 | Phase 2 |
NCT01677286 | Boston University | Amyloidosis | July 2012 | Phase 2 |
NCT00511875 | Thomas Gardner|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetic Retinopathy | July 2008 | Phase 2 |
NCT04108897 | Johns Hopkins University | Rosacea | September 17, 2019 | Early Phase 1 |
NCT00631501 | Kaunas University of Medicine|University Hospital, Linkoeping | Lateral Epicondylalgia (Tennis Elbow) | Not Applicable | |
NCT02203682 | Sun Yat-sen University | Graves Ophthalmopathy|Graves Disease|Eye Diseases|Thyroid Diseases|Endocrine System Diseases|Eye Diseases, Hereditary|Hyperthyroidism|Autoimmune Diseases|Immune System Diseases | July 2014 | Phase 2 |
NCT02005653 | Indian Council of Medical Research | Filarial; Infestation | February 2009 | Phase 4 |
NCT03585140 | Centro Dermatológico Dr. Ladislao de la Pascua | Acne Vulgaris|Diet Modification | January 1, 2016 | Not Applicable |
NCT02147262 | University Medical Centre Ljubljana|University of Ljubljana School of Medicine, Slovenia|Medical University of Vienna|Harvard University | Chronic Atrophic Acrodermatitis | July 2013 | Not Applicable |
NCT02220751 | University of Sao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontitis|Type 2 Diabetes Mellitus | March 2009 | Phase 3 |
NCT01825408 | University of North Carolina, Chapel Hill | Sinusitis | February 2013 | Phase 4 |
NCT02884713 | King Faisal Specialist Hospital & Research Center | GASTRITIS | June 2013 | Not Applicable |
NCT02726646 | University of Campinas, Brazil|Pontificia Universidade Catolica de Sao Paulo | Chronic Periodontitis | June 2015 | Phase 2 |
NCT00883818 | Samsung Medical Center | Overactive Bladder | January 2007 | Phase 4 |
NCT00829790 | Teva Pharmaceuticals USA | Healthy | October 2006 | Phase 1 |
NCT01949233 | University of Oxford|Oxford University Hospitals NHS Trust | Marfan Syndrome | October 2013 | Phase 2 |
NCT01518192 | University Medical Centre Ljubljana|Slovenian Research Agency | Erythema Migrans|Post-Lyme Disease Symptoms | June 2006 | Phase 4 |
NCT02845024 | Islamic Azad University, Tehran | Diabetes Mellitus With Periodontal Disease | September 2014 | Not Applicable |
NCT01879930 | University Hospital Inselspital, Berne | Chronic Pelvic Pain Syndrome|Bladder Pain Syndrome | November 2012 | Phase 4 |
NCT00041977 | CollaGenex Pharmaceuticals | Acne Rosacea | June 2002 | Phase 3 |
NCT02341209 | Rochester General Hospital | Cutaneous T-cell Lymphoma|Mycosis Fungoides|Sezary Syndrome | February 6, 2018 | Phase 2 |
NCT00002872 | Eastern Cooperative Oncology Group|National Cancer Institute (NCI)|North Central Cancer Treatment Group | Metastatic Cancer | November 1996 | Phase 3 |
NCT03162497 | Medical University of Vienna | Dry Eye Syndromes|Meibomian Gland Dysfunction | January 8, 2018 | Phase 4 |
NCT01418742 | Gesellschaft fur Medizinische Innovation ? Hamatologie und Onkologie mbH|ClinAssess GmbH | Colorectal Carcinoma | August 2011 | Phase 2 |
NCT00980148 | National Institute of Allergy and Infectious Diseases (NIAID) | Chlamydial Infection | December 2009 | Phase 3 |
NCT03342456 | The Third Xiangya Hospital of Central South University|Livzon Pharmaceutical Group Inc.|Yung Shin Pharm. Ind. Co., Ltd. | Duodenal Ulcer Due to Helicobacter Pylori | December 13, 2017 | Phase 4 |
NCT04310930 | The University of Queensland|Australian Government Department of Health|Children´s Hospital Foundation|Cystic Fibrosis Foundation|Newcastle University|Griffith University|Erasmus Medical Center|Monash University|University of Copenhagen|Hôpital Cochin|South Australian Health and Medical Research Institute|University of Melbourne|James Cook University, Queensland, Australia|Murdoch Childrens Research Institute | Pulmonary Disease Due to Mycobacteria (Diagnosis) | March 2020 | Phase 2|Phase 3 |
NCT03709459 | Kirby Institute|South Australian Health and Medical Research Institute|Monash University | STIs Prevention | December 17, 2019 | |
NCT04067011 | Emergent BioSolutions|Biomedical Advanced Research and Development Authority | Anthrax | August 12, 2019 | Phase 2 |
NCT02844634 | British Columbia Centre for Disease Control | HIV|Syphilis | May 15, 2018 | Phase 4 |
NCT00647959 | Mylan Pharmaceuticals | Healthy | March 2006 | Phase 1 |
NCT00170222 | Medical Center Alkmaar | Chronic Obstructive Pulmonary Disease | July 2002 | Phase 4 |
NCT03075891 | Galderma | Rosacea | July 5, 2017 | Phase 4 |
NCT00031499 | National Institute of Allergy and Infectious Diseases (NIAID) | Syphilis | June 2000 | Phase 3 |
NCT01205464 | Linkoeping University | Fatigue|Radicular Pain|Cognitive Dysfunction|Paresthesia|Paresis | February 2005 | Not Applicable |
NCT01301586 | Nexgen Dermatologics, Inc. | ACNE VULGARIS | November 2010 | Phase 1|Phase 2 |
NCT02305940 | Imperial College London | Chronic Obstructive Pulmonary Disease (COPD) | July 2014 | Phase 3 |
NCT00351182 | Dong-Min Kim|Chosun University Hospital | Scrub Typhus | September 2005 | Phase 3 |
NCT03334682 | Nantes University Hospital | Acne Vulgaris | January 31, 2018 | Phase 3 |
NCT01788215 | University of Rochester | Polycystic Ovarian Syndrome (PCOS)|Irregular Menstrual Cycles|Androgen Excess | November 2010 | Phase 3 |
NCT03076281 | Sidney Kimmel Cancer Center at Thomas Jefferson University|Thomas Jefferson University | Larynx|LIP|Oral Cavity|Pharynx | April 3, 2017 | Phase 2 |
NCT00439166 | Hamilton Health Sciences Corporation|The Physicians´ Services Incorporated Foundation|McMaster University | Alzheimer´s Disease | February 2007 | Phase 3 |
NCT02463942 | University Medical Centre Ljubljana|University of Ljubljana School of Medicine, Slovenia | Tick-borne Encephalitis | September 2014 | Not Applicable |
NCT00803842 | Northwestern University | Non Small Cell Lung Cancer | October 2008 | Not Applicable |
NCT02086591 | University of Rochester | Adult Diffuse Large B-Cell Lymphoma|Mantle Cell Lymphoma Recurrent|Lymphoma, Follicular|Marginal Zone B-Cell Lymphoma|Malignant Lymphoma - Lymphoplasmacytic|Waldenstrom Macroglobulinemia|Small Lymphocytic Lymphoma|Chronic Lymphocytic Leukemia (CLL)|T-Cell Lymphoma | March 2014 | Phase 2 |
NCT03980223 | University of California, San Francisco|University of Washington|National Institute of Allergy and Infectious Diseases (NIAID)|Mayne Pharma International Pty Ltd|San Francisco Department of Public Health | Gonorrhea|Chlamydia|Syphilis | November 26, 2019 | Phase 4 |
NCT00355459 | University of Texas Southwestern Medical Center | Dry Eye Syndrome | August 2005 | Not Applicable |
NCT01254799 | Omar Mamdouh Shaaban|Assiut University | Uterine Hemorrhage | January 2008 | Phase 3 |
NCT01547325 | NanoSHIFT LLC|United States Department of Defense | Dehisced Surgical Wounds | May 2012 | Not Applicable |
NCT00653380 | Par Pharmaceutical, Inc.|Anapharm | To Determine Bioequivalence Under Fasting Conditions | September 1999 | Phase 1 |
NCT00635609 | Warner Chilcott | Acne Vulgaris | March 2008 | Phase 4 |
NCT03765931 | Institut de Recherche pour le Developpement | Fever | July 2016 | Phase 4 |
NCT01160640 | Harold Wiesenfeld|National Institute of Allergy and Infectious Diseases (NIAID)|University of Pittsburgh | Pelvic Inflammatory Disease | November 2010 | Phase 2 |
NCT01756833 | University of Maryland, Baltimore|National Institute on Aging (NIA) | Aneurysm | May 2013 | Phase 2 |
NCT00688064 | Galderma | Severe Acne Vulgaris | August 2008 | Phase 3 |
NCT01320033 | Galderma | Acne Vulgaris | March 29, 2011 | Phase 2 |
NCT03397004 | St. Michael´s Hospital, Toronto|Barrow Neurological Institute|Duke University|Feinstein Institute for Medical Research|University of Pittsburgh|Sunnybrook Health Sciences Centre | Hereditary Hemorrhagic Telangiectasia (HHT) | September 12, 2018 | Phase 2 |
NCT01635530 | Turku University Hospital | Lyme Neuroborreliosis | August 2012 | Phase 4 |
NCT03727620 | Mohammed V Souissi University | Aggressive Periodontitis | January 6, 2014 | Phase 1|Phase 2 |
NCT02688738 | Rothman Institute Orthopaedics | Propionibacterium | March 2015 | Not Applicable |
NCT00358462 | University of Washington|National Institute of Allergy and Infectious Diseases (NIAID) | Urethritis | January 2007 | Phase 3 |
NCT02864550 | British Columbia Centre for Disease Control | Syphilis|Sexually Transmitted Infections | August 15, 2019 | Phase 4 |
NCT01595594 | University of Sao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontal Disease|Type 2 Diabetes | March 2010 | Phase 3 |
NCT00964834 | PharmAthene, Inc.|National Institutes of Health (NIH)|Medarex|Quintiles, Inc.|Department of Health and Human Services | Anthrax | July 2009 | Phase 1 |
NCT01809444 | Sun Yat-sen University | Thyroid Associated Opthalmopathies | November 2012 | Phase 2|Phase 3 |
NCT01590082 | M.D. Anderson Cancer Center|National Institutes of Health (NIH)|National Cancer Institute (NCI) | Melanoma | November 2012 | Phase 1|Phase 2 |
NCT00207584 | Centers for Disease Control and Prevention | Mycoplasma Pneumoniae | January 1994 | Not Applicable |
NCT00775177 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Healthy | June 2005 | Not Applicable |
NCT03462329 | University Medical Centre Ljubljana | Erythema Migrans | June 1, 2018 | Not Applicable |
NCT00000403 | Indiana University|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|National Institute on Aging (NIA) | Osteoarthritis | September 1996 | Phase 3 |
NCT03508232 | University of Alberta|Royal Alexandra Hospital | ST Segment Elevation Myocardial Infarction|Heart Failure | January 6, 2020 | Phase 2 |
NCT02553473 | Sorlandet Hospital HF | Neuroborreliosis, Borrelia Burgdorferi | October 2015 | Phase 3 |
NCT02207556 | Medical College of Wisconsin | Primary Systemic Amyloidosis | October 1, 2014 | Phase 2 |
NCT01783106 | Royal Liverpool University Hospital|National Association for Colitis and Crohn´s Disease|National Institute for Health Research, United Kingdom | Crohn´s Disease | February 1, 2014 | Phase 2 |
NCT00353743 | Hospital de Clinicas de Porto Alegre | Abortion, Septic | May 2006 | Not Applicable |
NCT01727973 | Sun Yat-sen University | Graves Ophthalmopathy|Graves Disease|Eye Diseases|Thyroid Diseases|Endocrine System Diseases|Eye Diseases, Hereditary|Hyperthyroidism|Autoimmune Diseases|Immune System Diseases | October 2012 | Phase 1|Phase 2 |
NCT00857038 | Medical Center Alkmaar|Leiden University Medical Center|University of Amsterdam | Chronic Obstructive Pulmonary Disease|Inflammation|Pulmonary Emphysema | April 2009 | Phase 4 |
NCT02774993 | National University Hospital, Singapore|Tan Tock Seng Hospital|National University, Singapore|A*Star | Tuberculosis | September 2015 | Phase 2 |
NCT03474458 | IRCCS Policlinico S. Matteo | Cardiac AL Amyloidosis | February 11, 2019 | Phase 2|Phase 3 |
NCT02874430 | Sidney Kimmel Cancer Center at Thomas Jefferson University|Thomas Jefferson University | Breast Carcinoma|Endometrial Clear Cell Adenocarcinoma|Endometrial Serous Adenocarcinoma|Uterine Corpus Cancer|Uterine Corpus Carcinosarcoma | June 8, 2016 | Phase 2 |
NCT00016835 | University of Michigan|National Institute of Dental and Craniofacial Research (NIDCR) | Periodontal Disease|Diabetes Mellitus, Type 2 | October 17, 2001 | Phase 2 |
NCT00064766 | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Endometrial Bleeding|Periodontal Disease | February 2003 | Phase 4 |
NCT00803452 | University of Louisville | Blepharitis | July 2008 | Phase 4 |
NCT01434173 | Bayer|RTI Health Solutions | Drug-Induced Liver Injury | July 2001 | |
NCT00126204 | Barnes-Jewish Hospital | Aortic Aneurysm | March 2004 | Not Applicable |
NCT01917721 | Hawaii Pacific Health | Kawasaki Disease|Coronary Aneurysm | October 2013 | Phase 2 |
NCT02775695 | Medical College of Wisconsin | Resectable Pancreatic Cancer | April 3, 2017 | Phase 2 |
NCT03824340 | Aljazeera Hospital | Infertility | January 30, 2019 | Not Applicable |
NCT01847976 | Ottawa Hospital Research Institute|Canadian Breast Cancer Foundation | Pain | August 2013 | Phase 2 |
NCT02850913 | Makerere University|University of Oxford | Seizures | September 5, 2016 | Phase 2 |
NCT00764361 | NanoSHIFT LLC | Diabetic Foot Ulcer | January 2009 | Phase 2 |
NCT02036528 | Royer Biomedical, Inc. | Diabetic Foot Ulcers | January 2014 | Phase 1|Phase 2 |
NCT01661985 | Ostergotland County Council, Sweden|Statens Serum Institut | Urethritis|Cervicitis|Genital Mycoplasma Infection|Chlamydia Trachomatis | February 2010 | Phase 4 |
NCT01380483 | Par Pharmaceutical, Inc.|Anapharm | To Determine Bioequivalence Under Fasting Conditions | January 2000 | Phase 1 |
NCT00648180 | Mylan Pharmaceuticals | Healthy | July 2005 | Phase 1 |
NCT01426269 | Galderma Laboratories, L.P. | Rosacea | September 2011 | Phase 4 |
NCT02753426 | University of California, San Francisco | Chronic Kidney Disease|Cardiorenal Syndrome | April 2016 | Phase 1 |
NCT02583282 | Postgraduate Institute of Medical Education and Research | Malignant Pleural Effusion | August 1, 2015 | Not Applicable |
NCT02927496 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema|Lymphatic Filariasis|Filariasis | June 19, 2018 | Phase 3 |
NCT00652795 | Par Pharmaceutical, Inc.|Anapharm | To Determine Bioequivalence Under Fasting Conditions | July 2004 | Phase 1 |
NCT03956212 | University Medical Centre Ljubljana|University of Ljubljana School of Medicine, Slovenia | Erythema Migrans | June 1, 2017 | Not Applicable |
NCT00855595 | Bayer | Papulopustular Rosacea | February 2009 | Phase 4 |
NCT03457636 | Derm Research, PLLC | Acne | March 19, 2018 | Phase 4 |
NCT02894268 | Sir Run Run Shaw Hospital | Helicobacter Pylori Infection | February 2016 | Phase 4 |
NCT03465774 | M.D. Anderson Cancer Center|National Cancer Institute (NCI) | Malignant Pleural Effusion | March 8, 2018 | Early Phase 1 |
Chemical structure